Time-correlated Raman and fluorescence lifetime spectroscopy
Time. It cannot be measured. Sundials, dripping water, clocks, and radioactive decay are counters to other physical processes, but not time itself.
Time is everything in Raman spectroscopy. C.V. Raman himself took several days of exposing liquid benzene with a quartz mercury lamp in order to get the first Raman spectrum:
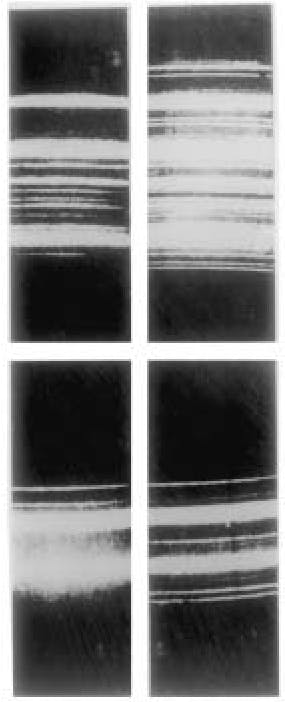
The first spectra taken by C. V. Raman and K. S. Krishnan. The upper-left photograph shows the incident light consisting of the spectrum of a quartz mercury arc lamp after passing through a blue-filter that cuts out all wavelengths greater than the indigo line at 4358 Angstroms. The upper-right photograph shows the same spectrum when scattered by liquid benzene and taken with a small Adam-Hilger spectroscope. Note the appearance of modified lines owing to the Raman effect. The lower-left and the lower-right photographs show the same effect using a different filter. (Courtesy of the Raman Research Institute, Bangalore.)
Recent laser experiments have revealed that an extremely small portion, approximately 1 in every 100 million parts, of light, undergoes a frequency change upon scattering. [1] Consequently, significantly lengthy exposure durations were necessary to capture spectra. For instance, Russian physicists who were also exploring the scattering of monochromatic light parallel to Raman, documented exposure times ranging from 2 to 14 hours and 100 hours (depending on the conditions) for crystals. [2] Subsequently, exposure times exceeding 180 hours were needed for vapours. [3]
In Raman spectroscopy, time plays a crucial role in understanding and analysing the scattering of light by molecules. The interaction between light and matter in Raman spectroscopy occurs over extremely short time scales, providing valuable information about molecular vibrations, rotations, and other dynamic processes.
These dynamic processes are induced by a short incident pulse of light. Short-duration laser pulses are typically used in Raman spectroscopy to probe molecular vibrations. The duration of the pulse must be shorter than the time scale of the molecular motion of interest to capture accurate Raman scattering signals. By controlling the ultrafast pulse duration, researchers can selectively investigate specific molecular motions and obtain detailed information about molecular structure and dynamics.

Sir CV Raman won the Nobel Prize in 1930. (Photo: Express archives)
Today, due to advancements in ultrafast electronics integrated into the detection of Raman signals, a clear spectrum can be produced in just a few seconds (depending on the material). I believe it would absolutely shock Raman and his collaborators (taking days to produce a clear signal) if they knew just how advanced this field has become within the last century.
Furthermore, there have been increasingly positive developments on the front of time-correlated Raman spectroscopy in regards to discriminating the fluorescence spectrum. Heather McEwan (MPhys), at the University of Edinburgh, is employing an ultrafast single photon detector, the Single Photon Avalanche Diode (SPAD), to spatially resolve chemical elements across a sample with a diminished fluorescence. [4] Currently, she is exploring avenues in the medical field where she has enhanced the chemical fingerprint of liver tissue, which is being screened for transplants which would otherwise be rejected or misused.
In Raman spectroscopy, a major factor in signal noise and interference is fluorescence. Following the non-radiative processes, the excited electrons can undergo radiative decay once they have absorbed energy, leading to the emission of fluorescence photons. This radiative decay occurs when the excited electrons transition back to lower energy levels, releasing the excess energy as photons. The time delay between excitation and the detection of fluorescence depends on the specific properties of the sample, such as its molecular structure, environment, and the presence of any quenching agents.
In some cases, the fluorescence emission occurs (relatively) with very long time delays, on the order of nanoseconds. This is often observed in fluorescent dyes or molecules with highly efficient radiative decay pathways. However, there can also be cases where the fluorescence emission is delayed further. This delay may be due to factors such as the presence of non-radiative relaxation processes that compete with radiative decay, or the presence of long-lived excited states that take longer to decay.
Additionally, fluorescence lifetime is a characteristic parameter that describes the average time it takes for a fluorophore to transition from the excited state to the ground state through radiative decay. The fluorescence lifetime can range from picoseconds to microseconds, depending on the specific fluorophore and its environment.
In Raman scattering, the photons interact with the sample's molecules and cause them to undergo vibrational or rotational transitions. This interaction results in a shift in the energy of the scattered photons, known as the Raman shift, which corresponds to the energy differences associated with the molecular vibrations or rotations.
The time delays associated with Raman scattering depend on several factors. Firstly, there is typically a very short delay, on the order of femtoseconds to picoseconds, between the interaction of the incident photons with the sample and the onset of the Raman scattering process. This delay is due to the time required for the laser photons to interact with the molecules and initiate the molecular vibrations or rotations.
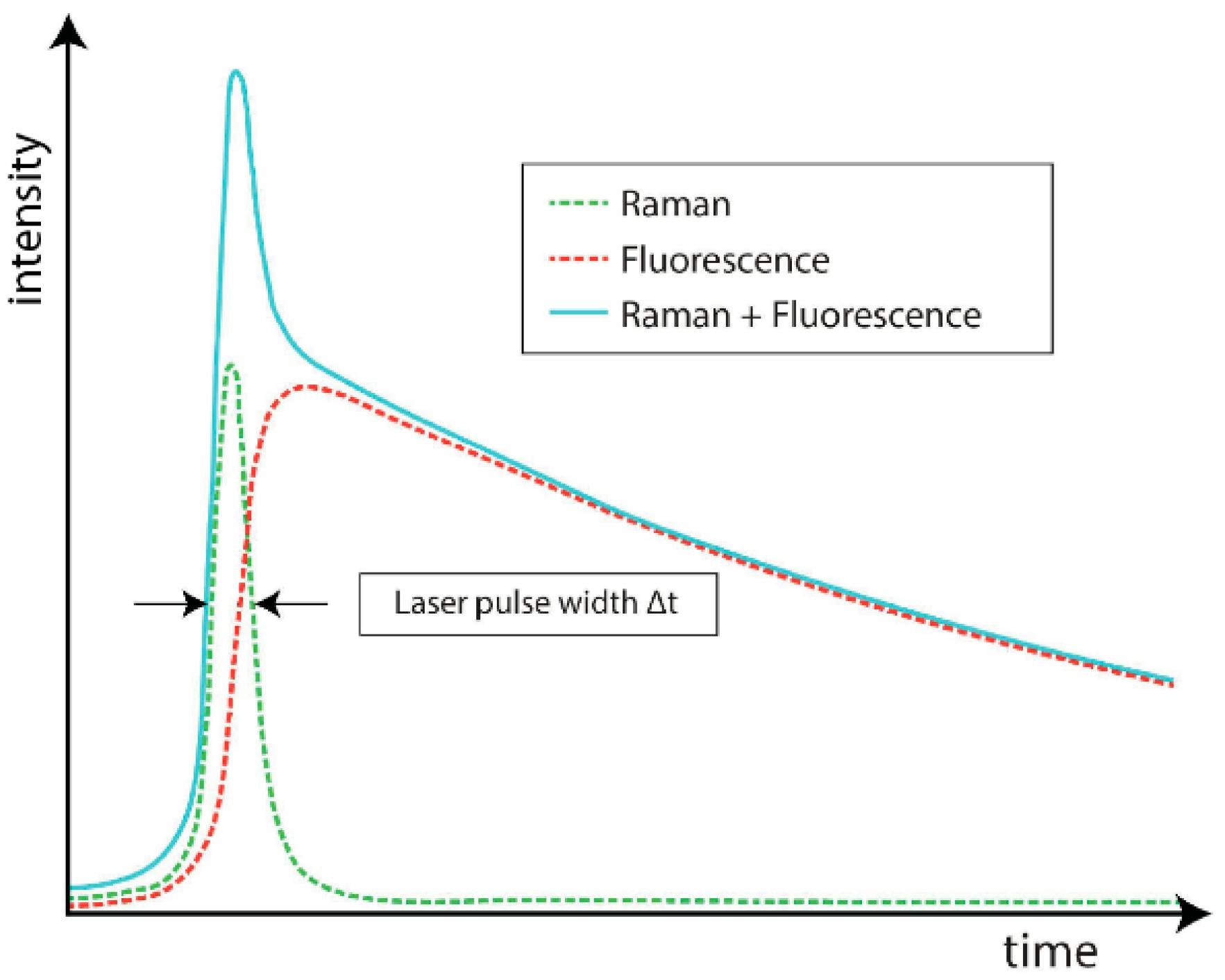
Scattering intensity as a function of time after the incident laser pulse. Note that the fluorescence signal intensifies with a short time delay from the Raman. [5]
The time difference between Raman and fluorescence signals can allow spectroscopists to discriminate the fluorescence pattern without perturbing the incident laser wavelength. By applying a picosecond time gate to the CCD, the Raman signal can be collected with reduced fluorescence.
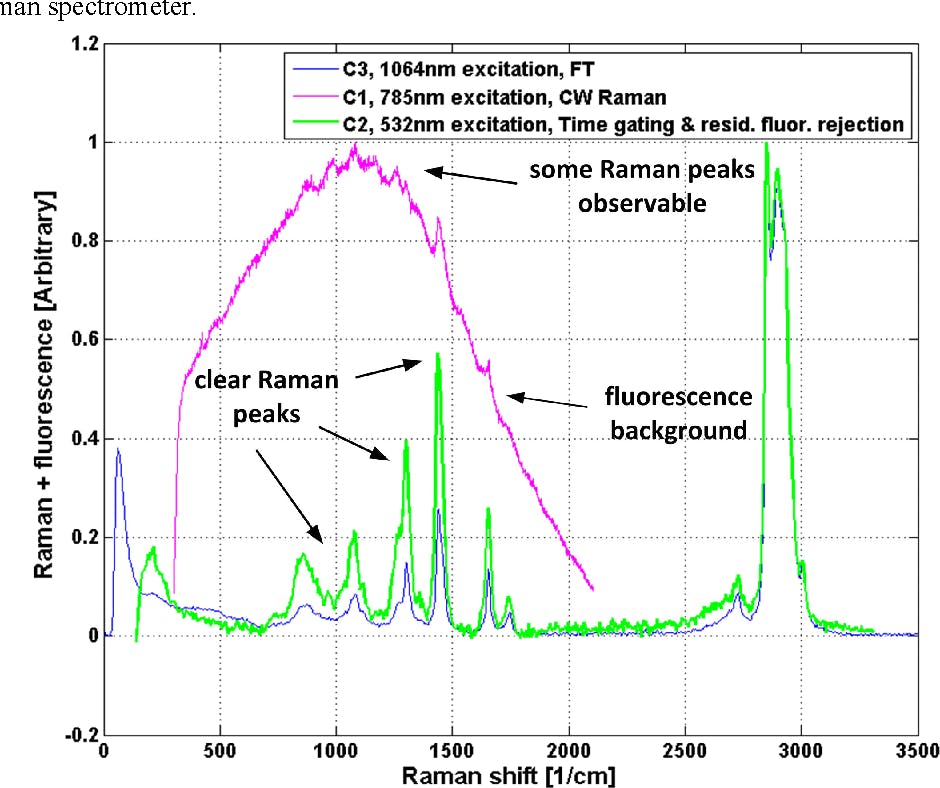
Raman spectra were recorded for an olive oil sample. The red curve (C1) is based on standard CW Raman measurement, the green curve (C2) is based on time-gated CMOS SPAD measurement, and the blue curve (C3) is based on FTIR measurement. [6]
This delay, known as the time delay or time window, allows for the separation of the Raman signal from the background noise and fluorescence. By carefully selecting the time delay, researchers can isolate the desired Raman signal and enhance the sensitivity and accuracy of the spectroscopic measurements.
Furthermore, time-resolved Raman spectroscopy is a specialized technique that provides information about the temporal evolution of molecular processes. It involves monitoring the Raman scattering over a range of time delays to capture dynamic changes occurring in the sample. Time-resolved Raman spectroscopy can reveal details about chemical reactions, energy transfer processes, and relaxation dynamics on picosecond to femtosecond time scales.
The advent of ultrafast laser technology has significantly advanced time-resolved Raman spectroscopy. It allows for the investigation of ultrafast processes and provides insights into molecular reactions and transient species that exist for only short periods.
CV Raman’s breakthrough was founded on a lot of discourse and theory on the properties and structure of light. Separated and distant in India from the discussion and hammerings in the West, Raman’s seemingly plane results of exposure lines on a screen sparked a revolution in chemical analysis and perhaps pushed the demand for high-powered lasers and advanced detector electronics.
I am excited to see what the future holds for these developments in Raman spectroscopy. Combing the multiple techniques in electrical engineering, laser physics, and chemical analysis could lead to the next breakthrough in improving Raman’s chemical fingerprint.
Only time will tell.
References
[1] - B. Chase, ‘‘A New Generation of Raman Instrumentation,’’ Applied Spectroscopy 48 (1994),14–19.
[2] - Landsberg and Mandelstam, ‘‘neue Erscheinung’’; ‘‘Lichtzerstreuung’’ (ref. 87).
[3] - Clemens Scha¨ fer and Frank Matossi, ‘‘Der Ramaneffekt,’’ in A. Eucken, ed., Fortschritte derChemie,Physik und Physikalische Chemie 20 (Berlin: Gebru¨ der Borbtreger, 1929), p. 22
[4] - Neil Finlayson, Heather McEwan, Gillian E. Brown, Alistair Gorman, Andrey Gromov, Colin J. Campbell, Ahmet Erdogan, Robert K. Henderson, Gareth O. S. Williams, "Time-correlated Raman imaging with a SPAD line sensor," Proc. SPIE 12373, Optical Biopsy XXI: Toward Real-Time Spectroscopic Imaging and Diagnosis, 1237302 (6 March 2023);
[5] - Madonini, Francesca, and Federica Villa. 2021. "Single Photon Avalanche Diode Arrays for Time-Resolved Raman Spectroscopy" Sensors 21, no. 13: 4287. https://doi.org/10.3390/s21134287
[6] - Kostamovaara, Juha T. et al. “Fluorescence suppression in Raman spectroscopy using a time-gated CMOS SPAD.” Optics Express 21 25 (2013): 31632-45.
