Overcoming Challenges in Raman Spectroscopy for Bioprocessing
Raman Spectroscopy Techniques in Mammalian Cell Bioreactors
Raman spectroscopy has emerged as a powerful tool for analyzing bioprocessing cells within bioreactors. By providing valuable insights into cellular composition, metabolic activity, and product quality, Raman spectroscopy enables real-time monitoring and optimization of bioprocesses. However, when it comes to obtaining Raman samples directly from bioreactors, several challenges must be addressed to ensure accurate and reliable data acquisition. In this article, we explore the unique obstacles faced in Raman spectroscopy for bioprocessing cells and discuss potential strategies to overcome them.
Challenge 1: Background Fluorescence
One of the major challenges in Raman spectroscopy of bioprocessing cells is the presence of strong background fluorescence. Biological samples often exhibit autofluorescence due to the intrinsic fluorescence properties of biomolecules, such as flavins, porphyrins, and NADH. This fluorescence can overwhelm the Raman signal, making it difficult to obtain clear and interpretable spectra. Additionally, the fluorescence emission can overlap with the Raman bands of interest, further complicating the analysis.
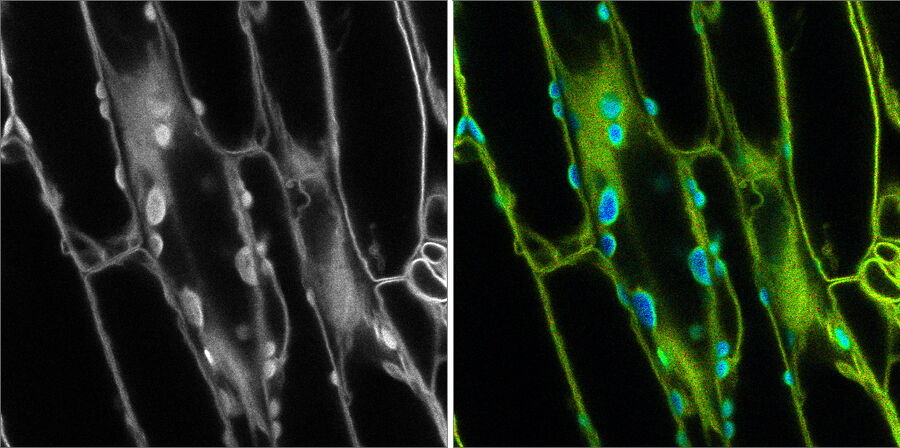
Figure 1: Fluorescence microscopy image on the left with no distinction between the fluorescent signal and background autofluorescence. FLIM was used in the image on the right to differentiate autofluorescence in chloroplasts (blue) from the desired fluorescent signal from the cell membrane (green) [Leica Microsystems]
To overcome background fluorescence, various techniques can be employed. One approach involves using excitation wavelengths in the near-infrared (NIR) region, as biological samples typically exhibit reduced fluorescence in this range. NIR excitation minimizes the interference caused by autofluorescence, allowing for more accurate detection of Raman signals. Additionally, advanced data processing techniques, such as background subtraction algorithms and multivariate analysis, can help to enhance the Raman signal and extract relevant information from the spectra.
Challenge 2: Sampling Complexity
The sampling of bioprocessing cells directly from bioreactors introduces additional complexities. Bioreactors often contain a heterogeneous mixture of cells, media, and other components, making it challenging to obtain representative Raman spectra from specific cell populations. The presence of suspended solids, gas bubbles, and varying turbidity can further interfere with the acquisition of high-quality Raman signals.

Figure 2: Overview of the flow cytometer. Sheath fluid focuses the cell suspension, causing cells to pass through a laser beam one cell at a time. Forward and side scattered light is detected, as well as fluorescence emitted from stained cells.
Techniques such as flow cytometry or other cell sorting methods can be employed to isolate specific cell populations before Raman analysis. By separating the cells of interest from the bulk mixture, a more accurate and targeted analysis can be performed. Flow cytometry allows for the identification and sorting of cells based on their specific characteristics, such as size, fluorescence, or other surface markers. Additionally, strategies like in situ Raman spectroscopy, where the Raman probe is placed directly in the bioreactor, can minimize sampling complexity and provide real-time monitoring of the bioprocess.
Challenge 3: Photodamage
Raman spectroscopy involves the use of laser light, which can potentially cause photodamage to sensitive bioprocessing cells. Excessive laser power or prolonged exposure can lead to cell death or alterations in cellular metabolism, thereby affecting the accuracy of the Raman analysis. Balancing the need for a strong Raman signal with minimal photodamage is crucial in bioprocess monitoring.
Optimal laser power and exposure time need to be carefully determined to minimize photodamage while still obtaining reliable Raman spectra. Lower laser power and shorter exposure times can be employed to reduce the risk of cell damage. It is important to select laser parameters that provide sufficient Raman signal intensity for analysis without compromising the viability and integrity of the cells. Additionally, the use of low-frequency Raman shifts or surface-enhanced Raman spectroscopy (SERS) techniques can enhance the Raman signal, allowing for lower laser power requirements.
Challenge 4: Bioreactor Environment
The bioreactor environment itself presents challenges for Raman spectroscopy. Factors such as temperature, pressure, and agitation can impact both the stability of the Raman signal and the viability of the cells. Temperature fluctuations and mechanical vibrations can introduce noise into the Raman spectra, making data interpretation more challenging. Additionally, the presence of complex media and varying nutrient concentrations in bioreactors can affect the Raman spectra and complicate the analysis.

To mitigate the effects of the bioreactor environment, it is important to carefully control and monitor the operating conditions. Stable temperature control systems and vibration isolation mechanisms can help minimize environmental disturbances and ensure consistent and reliable Raman measurements. Calibration and validation procedures should be implemented to account for any variations introduced by the bioreactor setup. Furthermore, careful consideration should be given to the choice of culture media and nutrient concentrations to minimize any interference with the Raman spectra.
Challenge 5: Data Interpretation and Analysis
Obtaining Raman spectra from bioprocessing cells is only the first step; extracting meaningful information from the spectra poses its challenges. Biological samples exhibit complex Raman spectra with numerous overlapping bands, requiring advanced data analysis techniques for accurate interpretation. Additionally, the presence of noise, baseline fluctuations, and spectral artefacts can hinder data analysis and make it difficult to identify specific biomolecular signatures.
Mitigation Strategy: Advanced data processing and analysis methods are essential for overcoming these challenges. Multivariate analysis techniques such as principal component analysis (PCA), partial least squares (PLS), and machine learning algorithms can be applied to extract relevant information from complex Raman spectra. These methods allow for the identification of characteristic spectral patterns associated with specific cellular components or metabolic states. Collaboration between spectroscopists and bioprocessing experts can also help in developing targeted algorithms and models for data interpretation.
Raman spectroscopy holds immense potential for analyzing bioprocessing cells within bioreactors, enabling real-time monitoring, and process optimization. However, the challenges associated with background fluorescence, sampling complexity, photodamage, bioreactor environment, and data interpretation must be addressed to obtain accurate and reliable data. By employing appropriate strategies such as NIR excitation, advanced data processing, cell sorting techniques, careful laser power optimization, and environmental control, these challenges can be overcome, paving the way for improved bioprocess characterization and control. With further advancements in Raman spectroscopy technology and methodologies, its integration into bioprocessing will continue to enhance our understanding of cellular behaviour, ultimately leading to more efficient and reliable bioproduction processes.
In recent developments, Raman spectroscopy will be used to establish key insights into bioprocessing. To ensure efficient and high-quality manufacturing, it is essential to monitor and optimize the cellular behaviour and metabolic processes within the bioreactor. Raman spectroscopy techniques have emerged as valuable tools for achieving these goals.
Cell Monitoring: Raman spectroscopy enables non-invasive and real-time monitoring of mammalian cells in a bioreactor. It provides information about the cellular composition, molecular structure, and biochemical changes occurring within the cells. This monitoring is crucial for understanding cell behaviour, growth, and metabolism during bioprocessing.
Process Optimization: Raman spectroscopy can help optimize bioprocesses by providing insights into cell viability, nutrient consumption, waste production, and other critical parameters. By monitoring these parameters, scientists can make informed decisions to optimize the culture conditions, media composition, and process parameters, leading to improved cell growth, productivity, and product quality.
Quality Control: Raman spectroscopy allows for the assessment of cell quality and product integrity in real time. It can detect changes in cellular composition and identify any deviations from normal metabolic activities. By monitoring the cells continuously, it is possible to identify potential issues early on, enabling corrective measures to be taken promptly, thus ensuring the production of high-quality products.
Process Understanding: Raman spectroscopy provides detailed molecular information about the cells, including DNA, proteins, lipids, and metabolites. By analysing the Raman spectra, scientists can gain insights into cellular processes, such as protein folding, lipid metabolism, and intracellular signalling pathways. This understanding is valuable for optimizing cell culture conditions, developing new cell lines, and enhancing bioproduction processes.
Scale-up and Bioprocess Development: Raman spectroscopy can aid in the scale-up of bioreactor processes by providing real-time data on cell behaviour and metabolic activity at different scales. This information helps in determining the optimal conditions and strategies for large-scale production. Additionally, Raman spectroscopy can assist in the development of new bioprocesses, allowing researchers to monitor and optimize the growth and behaviour of mammalian cells in various culture conditions.
Raman spectroscopy techniques have become indispensable tools in the field of bioprocessing, particularly in the cultivation of mammalian cells within bioreactors. By enabling non-invasive, real-time monitoring and analysis, Raman spectroscopy offers valuable insights into cell behavior, process optimization, quality control, and process understanding. It plays a crucial role in enhancing bioprocessing outcomes, ensuring high-quality product manufacturing, and advancing the development of novel bioproduction strategies. As the biopharmaceutical industry continues to evolve, Raman spectroscopy will undoubtedly remain a key component in optimizing bioprocessing and driving innovation in the field of mammalian cell-based therapeutics.